Catalyzed Precursor Mechanisms
Effects of various metals on dioxin formation from chlorinated phenol
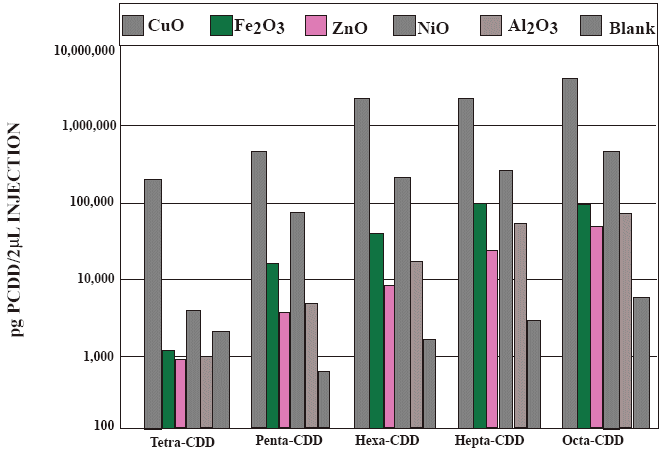
CONGENOR CLASS
|
Certain metals act as catalysts for dioxin formation, providing a surface upon which dioxins can readily form. This generally occurs during and after combustion processes on the fly ash in boilers and incinerators, but can also occur in other environments, such as in metals processing industries.
Copper (Cu) is the most potent catalyst for dioxin formation, but Iron (Fe), Zinc (Zn), Potassium (K) and Sodium (Na) have also been found in multiple studies to be correlated with increased dioxin/furan formation. Some studies have also indicated that Manganese (Mn), Magnesium (Mg) and Nickel (Ni) may also serve as catalysts for dioxin formation. Studies have conflicted on whether Aluminum (Al) encourages or inhibits dioxin formation. One study below indicated that Silicon (Si) is negatively correlated with dioxin formation. The metal catalyst issue is the likely reason why secondary copper and aluminum smelters are among the largest known sources of dioxin pollution in the U.S. Copper electrical wiring, coated with chlorine-containing PVC plastic is a perfect recipe for dioxin formation, when homes and buildings burn, when the plastic-coated wire gets burned in an incinerator, or when any of this plastic or its residues get into a secondary copper smelter. Other sources of dioxin pollution include metal-related industries with high temperature processes, such as iron ore sintering in the steel industry, aluminium recycling, copper ore melting, nickel refining, magnesium production, electrical cable splicing, and catalyst regeneration in the petroleum refining industry. [Kobylecki] Dioxin/furan formation during any natural or human activity requires three basic ingredients: an organic starting material, a chlorine source, and, in processes with relatively low temperatures, a metallic catalyst. [Kobylecki] |
Research has shown that synthesis of polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) during municipal waste combustion can proceed through a three step mechanism including 1) production of Cl2 from a metal-catalyzed reaction of HCl and O2, 2) Cl2 chlorination of aromatic rings through substitution reactions, and 3) formation of dual ring structures by a second metal-catalyzed reaction. Formation of the dual ring PCDD structure, likely through condensation reactions of chlorophenols, is enhanced up to three orders of magnitude in the presence of metal catalysts, such as Cu (II), reaching a maximum around 400°C.
Physical and chemical characteristics of fly ash samples from thirteen U.S. MSW incinerators were tested for correlations with PCDD concentrations. Strong correlations may indicate catalytic activity in the synthesis of PCDDs. Copper is strongly correlated with PCDD concentration but carbon and surface area are not. Other correlations include positive effects by sulfur, chlorine, sodium, potassium, and zinc and negative effects by silicon and aluminum.
Physical and chemical characteristics of fly ash samples from thirteen U.S. MSW incinerators were tested for correlations with synthesized PCDD concentrations. The fly ash was previously extracted to remove all PCDD. Pentachlorophenol was then passed over the samples in flowing nitrogen at 300°C. Strong correlations may indicate catalytic activity for PCDD synthesis. Copper, potassium, sodium, sulfur, and zinc were positively correlated and aluminum was negatively correlated. These elements closely match those identified in our earlier studies using PCDD concentrations of the raw fly ash. No correlations were noted for carbon, chlorine, or physical parameters such as surface area or particle size distribution.
In experiments with fly ash and model mixtures the formation conditions of PCDD/PCDF in the low temperaturede-novo-synthesis (300°) were investigated. Concentrations of sulfur dioxide, hydrogen chloride and elemental chlorine in the gas phase were varied. From the results it is concluded that in the reaction considered elemental chlorine does not play a decisive role. Furtheron the build-up of total organic chlorine (TOX), total extractable organic chlorine (EOX), as well as the oxidative degradation of particulate carbon and its conversion to carbon dioxide was studied. Regarding the involvement of metal ions, it is shown, that besides Cu(II) also Fe(III) ions may promote these reactions. From the experimental data it is proposed, that the de-novo-synthesis at 300°C from particulate carbon proceeds through a direct ligand transfer of the halide, in which metal ions (Cu, Fe) are involved.
The catalytic effects of copper and iron compounds were examined for their behavior in promoting formation of chlorine (Cl2), the major chlorinating agent of polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), in an environment simulating that of municipal waste fly ash. Formation of Cl2 occurred as a result of a metal-catalyzed reaction of HCl with O2. Catalytic activity was greatest at a temperature of approximately 400 °C, supporting a theory of de novo synthesis of PCDDs and PCDFs on fly ash particles downstream of waste combustion.
(cited in Kobylecki below as source for "dioxins can be created in the gas phase by reactions between aromatic rings containing chlorine (such as chlorobenzenes and chlorophenols) or by heterogeneous reactions between chlorinated organic precursors and fly ash based catalysts")
Experiments with model mixtures of carbon free fly ash or of Mg-Alsilicate with particulate organic carbon (charcoal, sugar coal, soot), potassium chloride and copper(II) chloride show that at 300° C in an air stream considerable amounts of PCDD/PCDF are produced. The yield depends on reaction time, the concentration of carbon and of copper(II) chloride and also on the presence of water vapor. Besides PCDD/PCDF also other organohalides are formed. From the results it is concluded that in municipal waste incineration particulate organic carbon is the primary source for PCDD/PCDF formation.
(cited in Addink below a source of "Copper has been identified as the strongest catalyst." statement)
The formation conditions of PCDD/PCDF on fly ash were studied in laboratory experiments. The increased formation of all congeners, as reported earlier, was confirmed for fly ash from different municipal waste incinerators. Essential for the PCDD/PCDF build up to occur is the presence of oxygen and of certain heavy metal/transition metal ions. From an investigation of the formation kinetics of the individual hepta-and hexa-CDD it is concluded that one of the processes involved in PCDD formation on fly as is a chlorination of organic precursor compounds.
The effect of thermal treatment of fly ash on the behaviour of PCDD/PCDF was studied in the range between 120 and 600° C. Annealing at 300° C (2 hrs) resulted in an increase of PCDD/PCDF concentration by a factor of 10 to 15. At 600° C degradation to concentrations below 0.1 ng/g is observed.
"However, flue gas and ashes from incinerators tend to contain unburnt carbon, heavy metals, and organic toxics, including dioxins. The dioxins can be created in the gas phase by reactions between aromatic rings containing chlorine (such as chlorobenzenes and chlorophenols) or by heterogeneous reactions between chlorinated organic precursors and fly ash based catalysts [Gullett 1990, Gullett 1992] as well as by de novo synthesis (i.e. complex reactions between unburnt carbon and chlorine sources with metallic catalysts, such as Cu, Fe, Mn, etc.). [Vogg 1986, Hinton 1991, Stieglitz 1989, Hagenmaier]"
Heavy metals can be grouped into various classes, each with its specific issues. Metals such as Cd, Cr, Hg and Pb are highly toxic. Cu, Pt and Ni tend to be less toxic but they are potent catalysts and contribute to a complex organic chemistry in the flue gases of combustion plants. In particular, they can contribute to the post-formation of dioxins in the flue gases.
"Copper (Cu) and nickel (Ni) tend to be less toxic than Cd, Hg or Pb, but they are potent catalysts and contribute to a complex organic chemistry in the flue gases of combustion plants. In particular, they can contribute to the post-formation of dioxins in the flue gases. In terms of recovery, Cu is undesirable in steel making but, along with Ni, it is potentially worth being recovered for use in the non-ferrous metals industry.Iron (Fe) and aluminium (Al) are less toxic and can also act as catalysts."
"Chlorine, copper, sodium, potassium, and zinc have a positive correlation with PCDD/F concentration, with copper being the most effective. Aluminum and silicon have a negative correlation.[Hinton 1991]""PCDDs and PCDFs are in this case probably formed from residual carbon present on the fly ash surface. Ions of heavy metals or of the transition metal group are essential for this formation reaction.[Stieglitz 1987] Copper has been identified as the strongest catalyst.[Vogg 1987]"
"Copper is very reactive in the formation of PCDDs and PCDFs. Various metal chlorides—such as MgCl2, ZnCl2, FeCl2, MnCl2, HgCl2, CdCl2, NiCl2, SnCl2, PbCl2, and CuCl2—have been tested for their ability to catalyze the formation of PCDD/Fs. From these metal chlorides, only CuCl2 made a significant contribution to the PCDD/F formation.[Stieglitz 1989] Without metal chlorides, only trace amounts of PCDD/Fs were found. The addition of only 0.08% Cu2+ gives rise to significant amounts of PCDD/Fs. The addition of 0.24 and 0.4% Cu2+ gives rise to an overproportional rise in PCDD/F formation.[Stieglitz 1989] It was later found that FeCl3 can catalyze the formation of PCDD/F too, but only when it is present in high amounts.[Stieglitz 1990]"
The following chart is from this presentation:
Catalyzed Precursor MechanismsEffects of various metals on dioxin formation from chlorinated phenol
CONGENOR CLASS |
|---|
Return to Dioxin Homepage
http://www.ejnet.org/dioxin/catalysts.html